α-tocopherol Metabolites
SP10 Endogenous metabolites of α-tocopherol as double-edged swords in the regulation of inflammatory stress
LCM Vit. E
Picture: FSUProject leaders:
Andreas Koeberle / Stefan Lorkowski
Doctoral candidate: Sijia Liao
Background and previous work
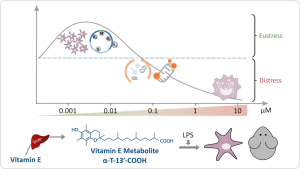
Immune cells trigger degenerative inflammatory conditions by their massive oxidative burst and excessive release of inflammatory mediators in response to exogenous stimuli such as LPS. α-Tocopherol (α-TOH) is used to prevent inflammation-associated diseases, although intervention studies in humans revealed contradictory results. Hepatic α-TOH catabolism results in an intermediate formation of the α-TOH long-chain metabolites (α-LCM) α-T-13'-OH and α-T-13'-COOH, which occur also in the circulation, as our group has shown. We further provided convincing evidence that α-LCM may comprise a new class of hormone-like regulatory metabolites. α-LCM time- and dose-dependently block the inflammatory response and oxidative burst in LPS-stimulated macrophages, likely by inducing adaptive stress response pathways. Moreover, α-LCM potently inhibit 5-lipoxygenase, a key enzyme for the biosynthesis of both pro-inflammatory and pro-resolving lipid mediators, which regulate immune cell survival, activity, differentiation and function in a hormetic and dynamic manner. Beside inflammation, α-LCM modulate lipid homeostasis, e.g., by inhibiting oxidised LDL uptake and lipid accumulation, due to reduced phagocytosis. Further preliminary studies show that the α-LCM induce DNA damage, but protect macrophages from lipotoxicity.
Specific aims and working programme
While an increasing number of reports add to the molecular mechanisms of α-LCM and show in vivo efficacy, the physiologically relevant mechanisms related to the regulation of inflammatory stress remained obscure. Our findings indicate α-LCM as lipokine-like signaling molecules that modulate adaptive stress responses in macrophages via pathways different from their metabolic precursor α-TOH. We propose that some of the actions of α-TOH in vivo are mediated, and are thus complicated, by endogenously formed α-LCM, in particular when α-TOH is supplemented. We are therefore exploring the actions of α-LCM (i) in vitro to obtain insights into the proposed adaptive stress response pathways, e.g., related to lipotoxicity, ER stress, autophagy and redox stress, and (ii) in murine disease models with inflammatory component. These proof-of-concept studies shall address the ability of α-LCM to protect mice from inflammatory stress by modulating adaptive stress responses and validate the molecular mechanisms of α-LCM in vivo by measuring appropriate serum and tissue parameters. A particular focus will be on (i) pathways involved in the induction of tolerance (i.e. the persistence response) or sensitization (i.e. the resistance response), and (ii) the identification of metabolipidomic determinants (e.g., membrane lipids and lipid mediators). Depending on the initial outcome, the physiological relevance of α-LCM-triggered pathways on stress regulation will be investigated by knockout, silencing and/or inhibitor approaches.