DNA methylation
Epigenetic stress response during calorie restriction in ageing intestine
Metabolism and Epigenetics
Graphic: Chao Lu et al., 2012Project leader: Franceso Neri
Doctoral candidate: Olena Husak
Dietary restriction (DR) remains the most reproducible and common anti-ageing intervention to extend lifespan and to improve health in mammals (Lee et al., 2016). In addition, DR can prevent tumorigenesis: meta-analysis of preclinical rodent models of multiple cancer types showed overall a 75.5% reduction in cancer incidence upon DR. (Ciara et al., 2017; Tammariello et al., 2010).
The incidence of gastrointestinal (GI) cancers drastically increases with age including colorectal cancer which is known to be associated with epigenetic aberrations, especially alterations in the DNA methylation pattern (Aguilera et al., 2006; Neri et al., 2015; Suzuki et al., 2004). Important to note, intestinal epithelium is an highly proliferative tissue deriving from the intestinal stem cells which are prone to accumulate genetic and epigenetic errors (Luebeck et al., 2019; Jean-Pierre Issa, 2014). There is an evidence that DR delays ageing at epigenetic level, particularly under reduced calorie intake mouse and primate models show delayed ageing associated epigenetic drift (Hahn et al., 2017; Shinji Maegawa et al., 2017; John J et al., 2017).
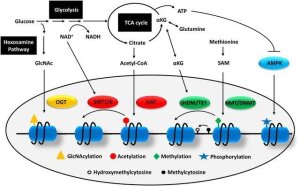
Molecular basis of DR-mediated effect up to date is known to be due an activation of nutrient sensing and stress response pathways which increase metabolic fitness, reduce inflammation and ultimately extend lifespan (Lopez-Lluch et al., 2016). The contributing role of epigenetic changes in DR is still not clear, however there is a possible link between metabolism and epigenetics. The enzymes participating in fine-tuning of epigenetic state of the cell are highly dependent on a number of metabolic intermediates: such as DNA and histone methyltransferases use S-adenosyl methionine (SAM) as the methyl donor. Acetyl-CoA is an essential substrate for acetylation of histone residues carried out by histone acetyl transferases. Other metabolites such as alpha-ketoglutarate is critical cofactor for the activity of DNA and histone demethylases (Chao Lu et al., 2012, Fig.1).