Liver Disease tolerance in Sepsis
SP9 Mechanisms of Disease tolerance in Sepsis-associated liver failure: Preventing progression of infection to sepsis with organ failure
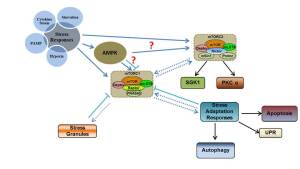
Role of mTOR in the inflammatory stress adaptation of hepatocytes
Graphic: Hussain, UKJProject leader: Michael Bauer / Ignacio Rubio
Doctoral candidate: Iqra Hussain
Background
Strategies to prevent or resolve organ damage harbor great potential to improve treatment of infectious diseases in the light of upcoming threats such as antibiotic resistance. The recently proposed concept of “disease tolerance” describes defense reactions involving an increased robustness and reduced vulnerability of parenchyma against infectious insults. Disease tolerance works hand-in-hand with immune resistance as the classical, antimicrobial reaction to infection managed by the immune system. A concerted balance between resistance and disease tolerance is key for an efficient adaptive host response. In contrast, excessive activation of resistance pathways causes hyperinflammation and immunopathology while inappropriate disease tolerance might entail tissue damage by fostering propagation of the infection. Hence, mechanisms governing these reactions and especially their cross-talk are of particular interest to find novel approaches to sepsis therapy.
Specific aims and working program
We will investigate the liver as a prototypical organ critically affected in sepsis and systemic inflammation. The extent of tissue damage in response to septic insults will be assessed employing various experimental models: hepatocyte/immune cell co-culture systems, organoid liver-on-chip approach and a preclinical animal model of septic peritonitis. To understand the regulation of disease tolerance we will apply genetically and pharmacologically modified monocytes and/ neutrophils to each of the models and investigate the effect on liver tissue damage using manifold readouts of liver dysfunction such as the well characterized phenotype of excretory failure. We will, in particular, place a focus on three major pathways previously linked to immune cell adaptation: the metabolic master pathway PI3K/Akt/mTORC1, the energy sensor AMPK and the Ras/Erk pathway which is a key regulator of cytokine release in myelomonocytic cells. Our laboratory has access to genetic or pharmacological strategies for the modulation of each of the mentioned pathways. If a modulation of disease tolerance by the engineered immunity is observed, we will investigate the underlying communication and signaling response of the hepatocyte in detail. In addition to the study of the myeloid immune compartment, we will also assess the effect of mesenchymal stem cells (MSCs), a cell type with proven immunomodulatory properties that has achieved remarkable protection in preclinical sepsis models. MSCs and MSC supernatants will be initially provided by our external collaborator M. Möbius, Dresden.
Finally, samples from prognosis-stratified animal models of sepsis and frozen samples from patients might allow proof of the concept of the implication of the mTORC1, AMPK and Ras/Erk signaling pathways, both in immune and parenchymal cells, in the continuum from infection to organ failure.