sphingosine 1-phosphate (S1P)
SP23 The role of sphingosine 1-phosphate (S1P) signalling in genotoxic stress-induced protection from septic shock
SP23_S1P
Picture: Gräler / UKJProject leader: Markus Gräler
Doctoral candidate: Anke Ziegler
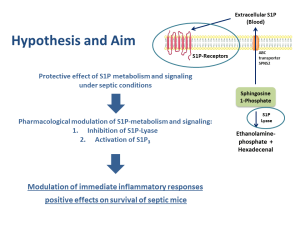
Sphingolipids are actively involved in cell fate decisions particularly in the presence of genotoxic stimuli. Our previous results indicate that the S1P-lyase is downregulated by genotoxic stress, which leads to an increase of S1P in local tissue. Increased S1P is able to activate S1P receptors. While the expression of the S1P receptor type 1 (S1PR1) is usually downregulated during infections, S1PR3 expression remains stable. We found out that stimulation of S1PR3 is able to dampen cytokine release and inflammation-induced pernicious levels of autophagy via downstream signaling of mitogen-activated protein kinases p38 and ERK. In addition, the lung epithelial barrier is stabilized. These effects were largely absent in S1PR3 deficient mice, demonstrating that activation of S1PR3 plays an important role for the observed increased disease tolerance.
Based on these data, we postulate that inhibition of the S1P-lyase and/or specific activation of S1PR3 might be valuable approaches for the prevention and/or treatment of sepsis. Therefore we are currently testing an inhibitor of the S1P-lyase and a specific agonist for S1PR3 as potential treatment options for sepsis intervention. We use polymicrobial peritoneal contamination and infection (PCI) as an in vivo model in mice for experimental sepsis to further investigate the efficacy and underlying pathways of the proposed treatment options.